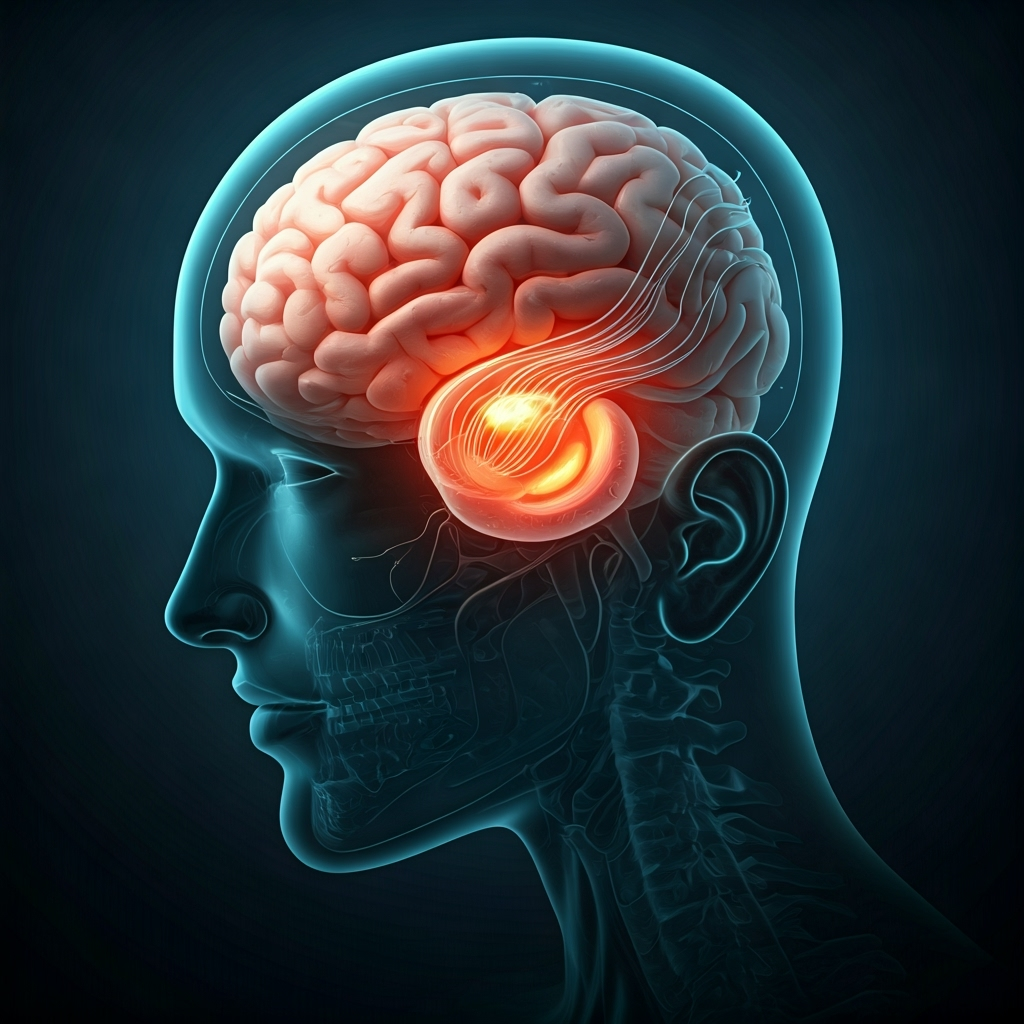
Elon Musk’s neurotechnology company, Neuralink, has received approval from the U.S. Food and Drug Administration (FDA) to proceed with a second human brain-chip implant. The clearance comes after the company identified and proposed a solution to a technical issue that affected its first-ever human trial participant, Noland Arbaugh.
Earlier this year, Arbaugh, a 29-year-old quadriplegic, successfully controlled a computer cursor using his thoughts after receiving the implant. However, several weeks after the surgery, a number of the device’s ultra-thin threads, which are embedded in the brain tissue, retracted, leading to a reduction in the device’s data-gathering capabilities. This diminished the implant’s effectiveness in translating neural signals into digital commands.
In response, Neuralink developed a software fix that enhanced the algorithm’s sensitivity, successfully recovering the device’s performance. For the upcoming second procedure, scheduled for June, the company plans to implement a more robust hardware solution. The new surgical protocol involves embedding the device’s threads eight millimeters deeper into the brain’s motor cortex, up from the initial three to five millimeters. The company believes this will prevent the threads from dislodging in the future.
This rapid turnaround—identifying a problem, developing a solution, and gaining regulatory approval for the next step—is a significant milestone for Neuralink. It demonstrates the company’s ability to iterate quickly in the highly complex and regulated field of brain-computer interfaces (BCIs). The progress with the PRIME (Precise Robotically Implanted Brain-Computer Interface) study is being closely watched as a potential breakthrough for millions of people living with paralysis, with the ultimate goal of restoring motor function and enabling control over digital technology through thought alone. The company aims to perform implants in ten people by the end of this year, pending further successful trials.